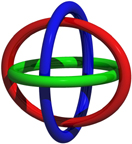
Borromean Rings:
An object of particular interest in knot theory is known as the Borromean Rings. It occurs in low-dimensional topology and is comprised (left) of three interlocked rings such that scission of any one ring leads to the other two falling apart. Although this symbol can be traced back to early Christian iconography and Norse mythology, its proliferation on crests and statues commissioned by the Borromeo family in fifteenth century Tuscany sealed its etymological fate. In addition to its having made cultural inroads into art and theology and heraldry, the last century witnessed its emergence on the scientific horizon in particle physics and magnetism, as well as in the formidable challenge it presents to synthetic chemists in search of its molecular expression.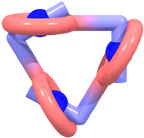
Daisy Chains:
The concept of threading a rod-shaped molecule through the macrocyclic cavity of another ring-shaped one, to create an interwoven host-guest complex (often referred to as a pseudorotaxane), can be applied to the propagation step of a supramolecular polymerization. Covalently coupling the two mutually-recognizing components to one another affords a self-complementary monomer, which has the capacity to self-assemble into either linear or cyclic (a cyclic trimer is illustrated left) daisy chain arrays. Furthermore, it can be envisioned that, unlike other supramolecular polymers, these aggregates, if appropriately functionalized, can subsequently be trapped kinetically, by under-going a 'stoppering' reaction, thus capturing an interlocked polymeric architecture, namely a polyrotaxane.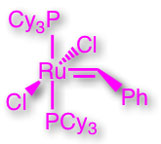
Olefin Metathesis and Dynamic Covalent Chemistry:
The final post-assembly modification steps employed in the majority of interlocked molecule syntheses are performed under kinetic control. Consequently, such strategies can result in the irreversible formation of undesired (non-interlocked) products, potentially reducing the efficiency of these processes. In contrast, however, a reversible thermodynamically controlled approach allows for a 'proof-reading' step in which 'incorrect' structures are consumed and their component parts recycled back into an equilibrating mixture. An ideal reaction that can be exploited for such ends, is the Ru-mediated olefin metathesis reaction using the catalysts (left) developed by Bob Grubbs at Caltech.