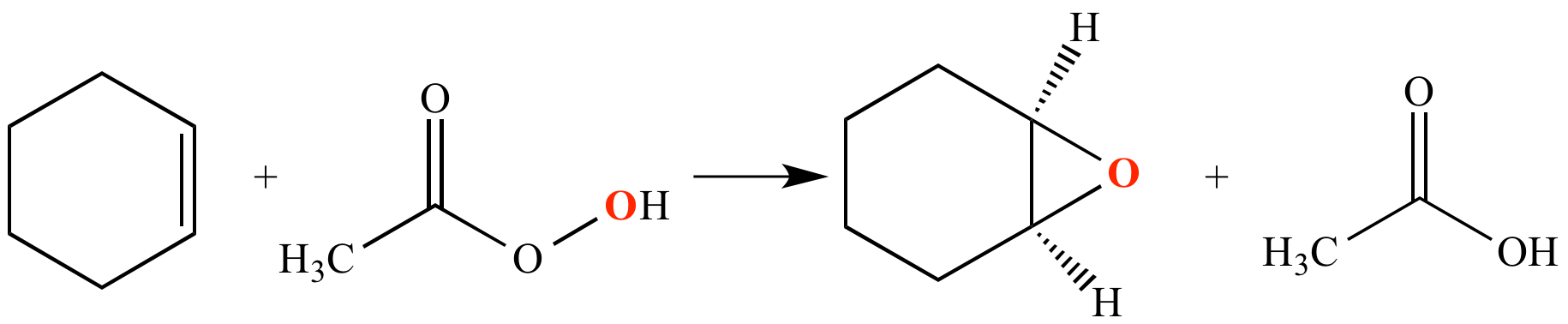
An alkene such as cyclohexene can be epoxidized by reaction with a peracid such as peracetic acid (CH3CO3H) or mCPBA. Alkene epoxidation by this method is an electrophilic addition reaction.
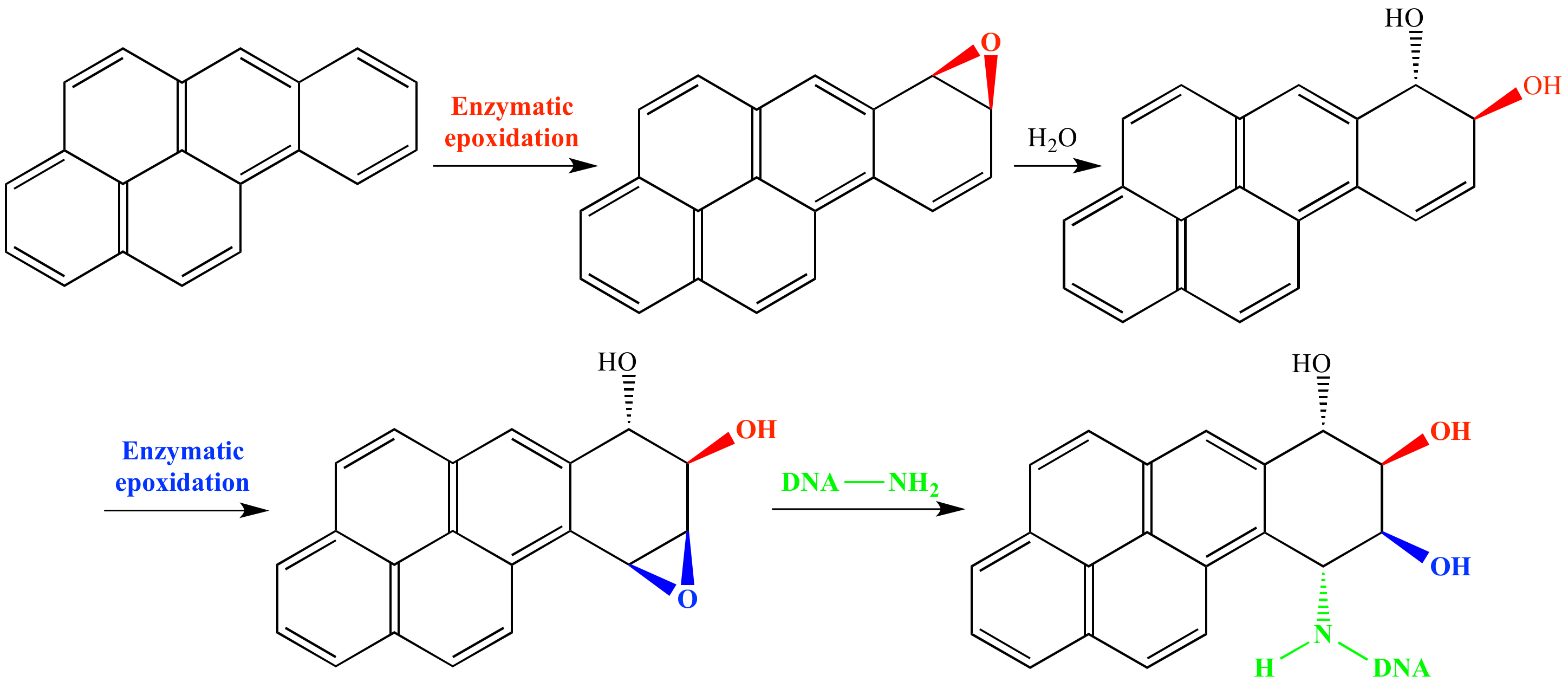
Benzo[a]pyrene is a human carcinogen. Benzo[a]pyrene is nonpolar so the body oxidizes it to increase its polarity and allow for faster excretion. In the liver benzo[a]pyrene is epoxidized by an enzyme, then hydrolyzed, and again epoxidized as shown above. The epoxide diol product is susceptible to nucleophilic addition by a guanine amino group in DNA, resulting in DNA alkylation, DNA mutation, and other problems.