In a covalent bond between two atoms of unequal electronegativity, the atom with higher electronegativity withdraws electron density towards itself, causing the δ+ and δ- charges of the bond dipole.
| Basicity: |
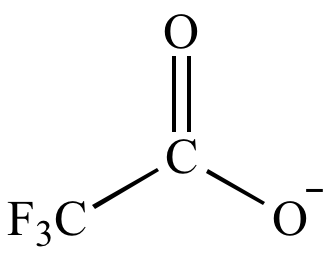 |
< |
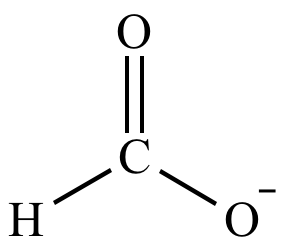 |
| Basicity: |
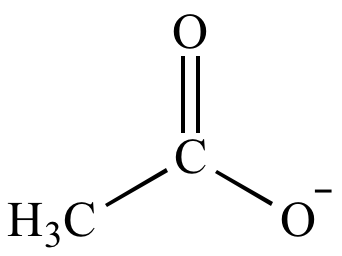 |
> |
 |