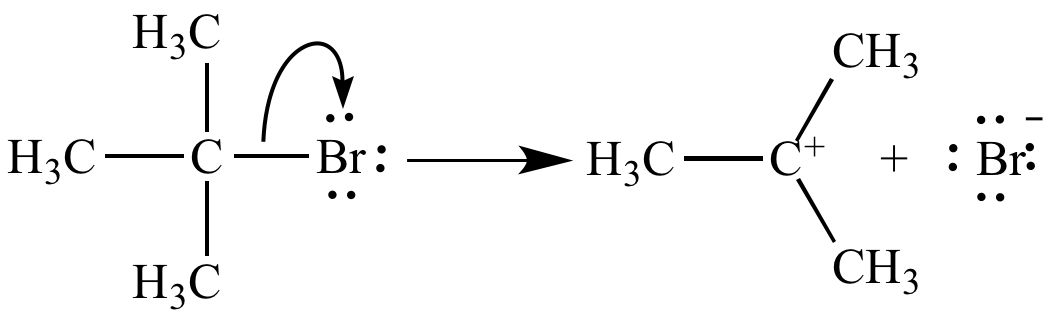
Ionization of tert-butyl bromide yields tert-butyl carbocation and bromide ion.
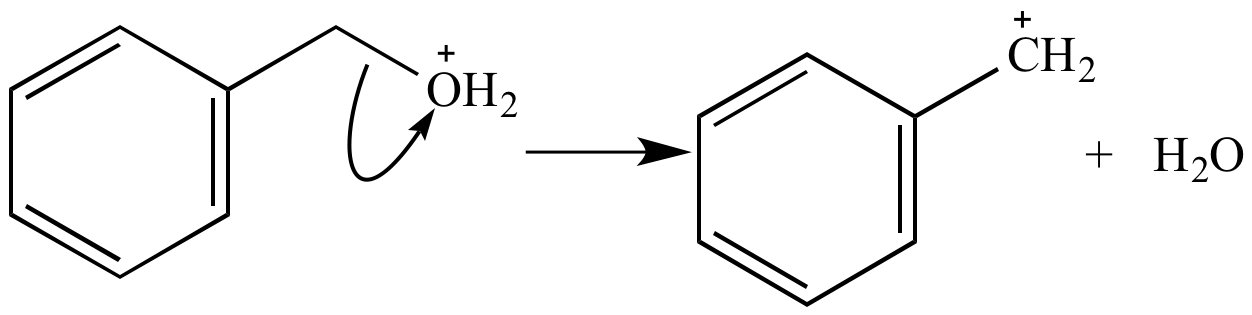
Ionization of this oxonium ion yields benzyl carbocation and a molecule of water.
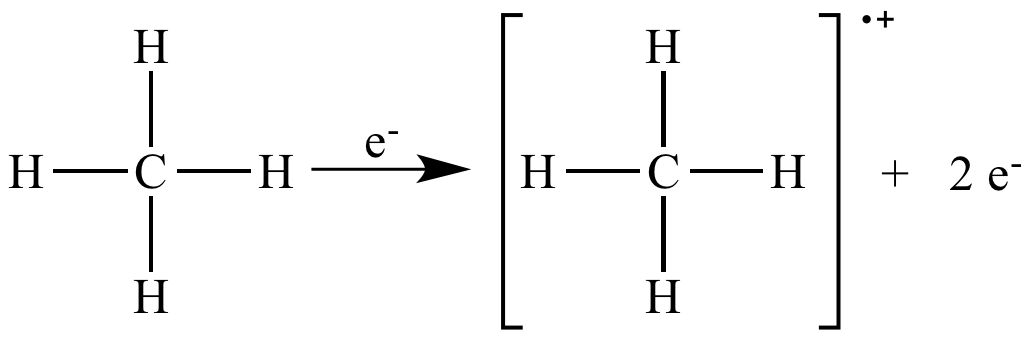
In mass spectrometry, electron impact ionization is a useful way to generate a radical cation molecular ion.