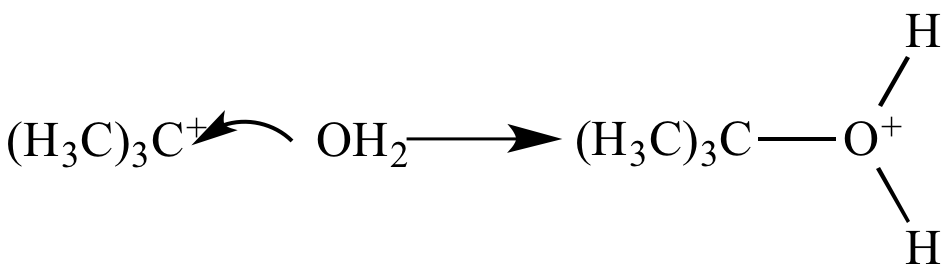
| Step 1: The carbon-chlorine bond
is ionized. |
|
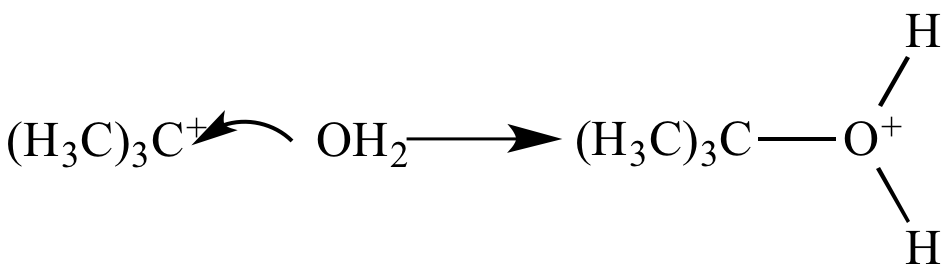 |
Step 2: The carbocation
captures
a molecule
of water,
and the carbon-oxygen bond
is formed. |
 |
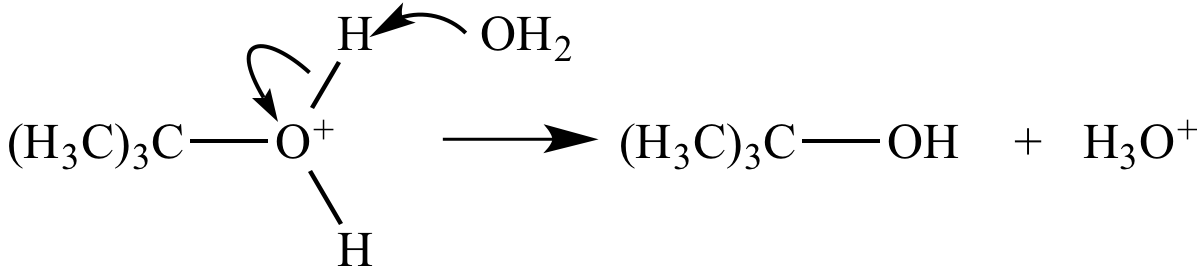
Step 3: The oxonium
ion is deprotonated
by water.
An oxygen-hydrogen bond
is broken, and separate oxygen-hydrogen bond
is formed. |