 Born-Oppenheimer approximation: we
cannot assume that nuclear motions of the solvent molecules are slow
relative to the electronic dynamics. In fact, it is the nuclear
motions of the solvent that mix the electronic energy levels together
and induce radationless transitions that allow the system to relax from
the electronic excited state. Fortunately, there are prescriptions
in the literature for dealing with the breakdown of the
Born-Oppenheimer approximation. All of these prescriptions make
uncontrolled assumptions about the nature of quantum decoherence -- as
of the current time, there is no rigorous way to treat non-adiabatic
dynamics in mixed quantum/classical systems (for more on decoherence in
quantum non-adiabatic molecular dynamics simulations, see B.
J. Schwartz et al., J. Chem. Phys. 104(15), 5942 (1996)).
In our group, we both take advantage of existing approaches for
non-adiabatic dynamics, and we develop new methods. For example, we
recently have extended an algorithm developed by others to be capable of
calculating non-adiabatic dynamics for multi-electron systems (before
our method, such calculations were limited to systems with only a single
quantum degree of freedom). We are now working to test our new
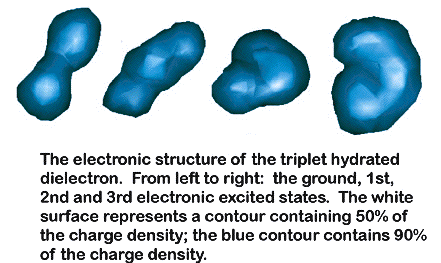
method and apply it to study systems such as solvated dielectrons (see
Figure above) as well as the alkali metal anions being studied
experimentally in our group via femtosecond spectroscopy. See,
e.g.,
Born-Oppenheimer approximation: we
cannot assume that nuclear motions of the solvent molecules are slow
relative to the electronic dynamics. In fact, it is the nuclear
motions of the solvent that mix the electronic energy levels together
and induce radationless transitions that allow the system to relax from
the electronic excited state. Fortunately, there are prescriptions
in the literature for dealing with the breakdown of the
Born-Oppenheimer approximation. All of these prescriptions make
uncontrolled assumptions about the nature of quantum decoherence -- as
of the current time, there is no rigorous way to treat non-adiabatic
dynamics in mixed quantum/classical systems (for more on decoherence in
quantum non-adiabatic molecular dynamics simulations, see B.
J. Schwartz et al., J. Chem. Phys. 104(15), 5942 (1996)).
In our group, we both take advantage of existing approaches for
non-adiabatic dynamics, and we develop new methods. For example, we
recently have extended an algorithm developed by others to be capable of
calculating non-adiabatic dynamics for multi-electron systems (before
our method, such calculations were limited to systems with only a single
quantum degree of freedom). We are now working to test our new
method and apply it to study systems such as solvated dielectrons (see
Figure above) as well as the alkali metal anions being studied
experimentally in our group via femtosecond spectroscopy. See,
e.g., R. E. Larsen and B. J. Schwartz, "An Efficient Real-Space Configuration-Interaction Method for Simulation of Multi-Electron Mixed Quantum/Classical Non-Adiabatic Dynamics in the Condensed Phase," J. Chem. Phys. 119(15), 7672-84 (2003).
C. J. Smallwood, W. B. Bosma, R. E. Larsen and B. J. Schwartz, "The Role of Symmetry in Charge-Transfer-to-Solvent Reactions: Quantum Non-Adiabatic Computer Simulation of Photoexcited Sodium Anions," J. Chem. Phys. 119(21), 11263-77 (2003).
This work is supported in part by the National
Science Foundation under award CHE-0204776.