

 |
Christopher S. FooteB.S. 1957, Yale University; Ph.D. 1962,
Harvard |
HonorsBanquet in Honor of Christopher S. Foote on his 65th Birthday RESEARCH INTERESTSThe organic and biological chemistry of molecular oxygen is of extraordinary interest. Oxygen plays an important role in aging, damage to materials in the environment, cellular pathology (for example, the damage following stroke or heart attack) and many other areas. The details of the chemical reactions underlying these processes are poorly understood. The Foote group uses preparative, physical-organic, and bioorganic methods to study the chemistry of molecular oxygen in photochemical and biological processes. Reactive intermediates include singlet oxygen (1O2, a metastable excited state of molecular oxygen), superoxide ion, and other oxygen species.1,2 |
|
|
| Singlet oxygen can be generated by many chemical and photochemical processes. A common way is by photochemical excitation of a sensitizer (Sens) to an electronically excited state (*Sens) followed by energy transfer from the excited sensitizer to oxygen. |
|
|
| Singlet oxygen is very toxic to organisms because it reacts with important biological molecules such as unsaturated lipids, oxidizable amino acids, and nucleic acids, particularly guanosine derivatives. The resulting reactions cause destruction of membranes, enzyme inactivation, and mutations, all of which can lead to cell death. We are carrying out extensive studies of the reactions with biological target molecules and have characterized many of the primary products of these reactions. For example, photooxidaton of guanosine derivatives gives endoperoxides that are stable only at low temperatures. This reaction is responsible for genetic damage caused by sensitizing molecules, light, and oxygen.3 |
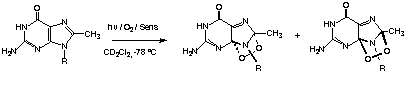 |
| Singlet oxygen also reacts with many organic compounds to give adducts which have utility in synthesis or are biological intermediates. Some examples are shown below.4-7 |
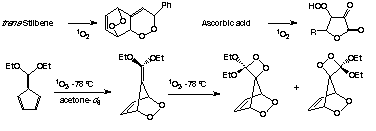 |
| Extensive mechanistic and preparative studies of the reactions of singlet oxygen are under way. For example, the reactions of indoles have been shown to depend critically on their structures. Some remarkable reactions of potential synthetic utility have been found; examples are shown below.8,9 |
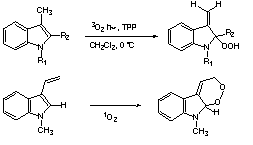 |
| Singlet oxygen can be conveniently observed by measuring its weak infrared emission using an ultrasensitive germanium diode detection system. If the sensitizer is excited by a short laser pulse, the emission can be followed as a function of time using a sophisticated system for computerized data acquisition and analysis. This technique allows a simple and accurate method of measuring the decay and reaction rates of this species as well as the efficiency of its production.10 |
|
|
| Reactive intermediates in these reactions are monitored by techniques that include laser flash photolysis, low temperature NMR spectroscopy, kinetic methods, and isolation. Some species characterized by such techniques in the last few years are shown below.11-17 |
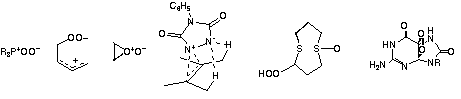 |
| A mechanistic example is the use of primary and secondary isotope effect studies to demonstrate the intermediacy of a perepoxide in the ene reaction of singlet oxygen with alkenes.18 A particularly interesting case involves trans-cyclooctene. This alkene, because of its strained and locked conformation, cannot readily undergo the ene reaction. Reaction with singlet oxygen leads to double bond isomerization, probably through a zwitterion formed from the intermediate perepoxide shown below. Triphenylphosphite stops the isomerization by trapping the intermediate. Reaction of trans-cyclooctene with the analogous enophile phenyltriazolinedione gives an analogous inter aziridine imide, which is stable enough to be characterzied by NMR at low temperatures.19 |
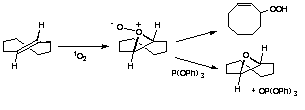 |
| A very important medical application of photosensitized oxidations is in photochemical killing of organisms. Oxygen-dependent photosensitized toxic effects are extremely common in nature, and are used, for example, by fungi to gain entry to cells by damaging protective membranes, or by plants to kill insects (or injure mammals) which feed on them. A particularly exciting application of this type of chemistry is the recent use of sensitizer, light, and oxygen to kill tumor cells selectively in humans.20 Studies of the mechanism of action of such sensitizers and attempts to prepare more effective ones are in progress. |
|
|
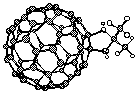
| Recently, we showed that the fascinating all-carbon molecules ("buckyballs") C60 and C70 have are powerful photosensitizers for the production of singlet oxygen, and their photophysical properties were characterized for the first time.21,22 Photochemical electron-transfer to and from these interesting molecules is possible.23,24 We also prepared some of the first stable characterizable derivatives, such as the dioxolane shown below.25 |
  |
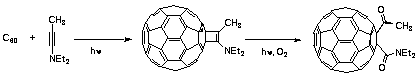
| The most efficient method so far of functionalizing C60 is photochemical [2 + 2] addition of the ynamine shown below to give the adduct cyclobutane enamine. The buckyball residue photosensitizes the oxidation of the enamine, and the difunctional adduct shown below is formed in 92% overall yield.26 |
 |
|
These are a few representative examples of ongoing work in this group. In summary, techniques from preparative and physical-organic chemistry and biochemistry are used as necessary to establish the role of key reactions of singlet oxygen or other species in chemical systems that are important to organic, biological, and medical science. |
|
1. Ferran Prat, K.N. Houk* and Christopher S. Foote*, "Effect of Guanine Stacking on the Oxidation of 8-Oxo-guanine in B-DNA," J. Am. Chem. Soc., 120, 845-846, (1998). 2. Ferran Prat and Christopher S. Foote*,
"A Resin-Bound Photosensitizer for Aqueous Photooxidations,"
Photochem. PhotoBiol., 67, 626-627 (1998).
|
updated on 2/13/03 by Alice Ramirez