

 |
Michael E. JungB.S. 1969, Rice University; Ph.D. 1973, Columbia University; NATO Postdoctoral, 1973-4, ETH, Zürich; Fulbright-Hays Senior Research Scholar,1980-8l; Alfred P. Sloan Fellow, 1979-8l; Camille and Henry Dreyfus Teacher-Scholar, 1978-83; UCLA Distinguished Teaching Award, 1978; Inaugural UCLA Gold Shield Faculty Prize, 1986-8; Glenn T. Seaborg Award, 1987; Fujii-Ohtsuka Professor, Tokushima University, 1991; UCLA McCoy Award, 1991-2; UCLA Hanson-Dow Teaching Award, 1992; American Chemical Society Arthur C. Cope Scholar Award, 1995.. |
|
|
|
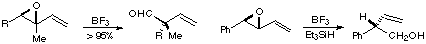
| Thus conversion of an aldehyde to the epoxy alcohol via Wittig reaction and reduction followed by Sharpless epoxidation generates the substrate for the rearrangement which is effected by treatment with trialkylsilyl triflates and base to give the protected aldol products in high yield. We are now extending this reaction to the synthesis of polypropionates by an iterative process so that compounds such as erythromycin and rifamycin could be prepared. |
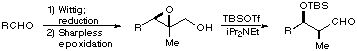
| We are also studying the use of radical cyclization-fragmentation processes for the synthesis of several natural products of biological interest. Finally, we are investigating the synthetic potential of substituent effects (gem-dialkyl, -dialkoxy, -dithioalkoxy, -dicarboalkoxy, bis(dialkylamino), etc.) and polar solvent effects on cyclizations, including the novel acceleration of the intramolecular Diels-Alder reactions of furfuryl methyl fumarates by the use of polar solvents. Dialkoxy substitution, both gem and vic, allows one to carry out a wide variety of cyclizations, including cyclizations to produce 4- and 8-membered rings. We are also studying the possibility of asymmetric induction in these processes. |
|
Jung ME, Kim WJ "Practical Syntheses of Dyes for Difference
Gel Electrophoresis" Bioorg. & Med. Chem., 2006,
14, 92-. Jung ME, Berliner JA, Angst D, et al. "Total synthesis
of the Epoxy Isoprostane Phospholipids PEIPC and PECPC"
Org. Lett., 2005, 7, 3933-3935. Jung ME, Jung ME, Min SJ, Houk KN, Ess D "Synthesis and relative stability of 3,5-diacyl-4,5-dihydro-1H-pyrazoles prepared by dipolar cycloaddition of enones and alpha-diazoketones J. Org. Chem., 2004, 69, 9085-9089 Jung ME, Maderna A "Microwave-assisted allylation of acetals with allyltrimethylsilane in the presence of CuBr " J. Org. Chem., 2004, 69, 7755-7757. Jung ME, Min SJ "Novel formation of a bridged bicyclic furan by rearrangement of a tetrahydroxydecalinone" Tet. Letts., 2004, 45, 6753-6755. Liew CK, Smith BT, Pilpa R, Suree N, Ilangovan U, Connolly KM, Jung ME, Clubb RT "Localization and mutagonesis of the sorting signal binding site on sortase A from Staphylococcus aureus" Febs Letts., 2004, 571, 221-226. Jung ME, Maderna "Allylation of acetals and ketals with
allyltrimethylsilane catalyzed by the mixed Lewis acid system
AlBr3/CuBr " Tet. Letts., 2004, 455301-5304.
Lansdown MG, Jung ME "Progress toward the total synthesis of Brasilicardin A" Abstracts Of Papers Of The Amer. Chem. Soc., 2003, 225, U351-U351 422-ORGN Part 2 Jung ME, Min SJ "Progress toward the synthesis of arisugacins and territrems" Abstracts of Papers of Amer., Chem. Soci., 2003, 226, U143-U143 190-Orgn Part 2 Wasserman JI, Jung ME "Progress toward the total synthesis of reticulidin A and deoxyreticulidin A" Abstracts Of Papers Of The Amer. Chem. Soc., 2003, 226, U146-U147 208-ORGN Part 2. Jung ME, Duclos BA "Diastereoselectivity in the Carroll rearrangement of beta-keto esters of tertiary allylic alcohols" Tet. Letts., 2004, 45, 107-109. Jung ME, van den Heuvel A "A tandem non-aldol aldol Mukaiyama
aldol reaction" Org. Letts., 5, 2003, 4705-4707 NOV
27
Connolly KM, Smith BT, Pilpa R, Ilangovan U, Jung ME, Clubb RT "Sortase from Staphylococcus aureus does not contain a thiolate-imidazolium ion pair in its active site" J. Biol. Chem., 2003, 278. Michael E. Jung,* Barbara Hoffmann, Bernhard Rausch, and Jean-Marie Contreras "Use of hindered silyl ethers as protecting groups for the non-aldol aldol process" Org. Letts., 2003, 5, 3159-3161. Jung ME, Pontillo J "Synthetic approach to potential
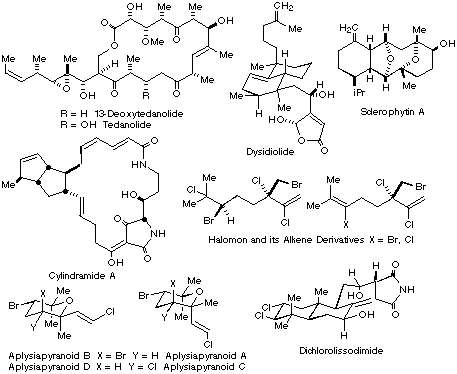
precursors of sclerophytin A" Tet., 2003
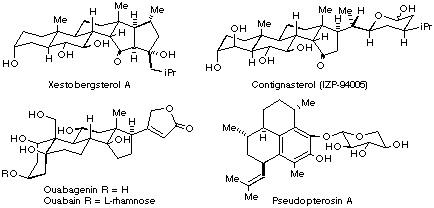
59. (15): 2729-2736. Jung ME, Piizzi G "Synthetic approach to the AB ring
system of ouabain Jung ME, Piizzi G "First synthesis of the A/B ring of
ouabain" Jung ME, Kers A, Subbanagounder G, Berliner JA "Studies Towards the Total Synthesis of an Epoxy Isoprostane Phospholipid, A Potent Activator of Endothelial Cells" Chem. Comms., 2003, 196-197 Jung ME, Davidov P "Efficient Synthesis of A Tricyclic BCD Analogue of Ouabain: Lewis Acid Catalyzed Diels-Alder Reactions of Sterically Hindered Systems" Angewandte Chemie-Int. Ed., 2002, 41, 4125-4128 Jung ME, van den Heuvel A "Diastereoselectivity in Non-Aldol
Aldol Reactions: Silyl Triflate-Promoted Payne Rearrangements"
Tet. Letts.. 2002, 43 , 8169-8172 . Jung ME, Pontillo J "Synthetic Approach to Analogues of the Original Structure of Sclerophytin A" J. Org. Chem., 2002, 67, (19): 6848-6851. Jung ME, van den Heuvel A "Tandem Non-Aldol Aldol Mukaiyama
Aldol Reaction" Abstracts of Papers Of The Amer. Chem.Soc.,
2002, 224: 541-ORGN Part 2.
|
|
Email: jung@chem.ucla.edu Phone: 310-825-7954 Fax: 310-206-3722 |
University of California, Los Angeles
Department of Chemistry and Biochemistry 607 Charles E. Young Drive East Los Angeles, CA 90095-1569 |
Updated on 8/29/06