Born 1963; Diploma in Chemistry, 1987, University of Fribourg, Sw itzerland; Ph.D. 1991, University of California, Los Angeles; Swiss National Science Foundation Postdoctoral Fellow, 1991-1992, Columbia University; Dreyfus New Faculty Award, 1992; NSF Young Investigator Award, 1994; Arnold and Mabel Beckman New Faculty Award, 1994.
Our research program uses modern synthetic organic chemistry to prepare and study new molecules designed to answer the most current and interesting problems in the biological and materials sciences. Interesting and often syn thetically challenging compounds are prepared which show unusual physical properties. We are presently working on two main areas which have recently gained tremendous significance.
1. Total Synthesis of Fullerenes: Since the isolation of the highly symmetrical all-carbon molecule buckminsterfullerene C60 (1), this new field of research has led to several major discoveries. One is the finding that the alkali metal salts K3C60 and Rb3C60 are superconductors with the highest critical transition temperatures ever achieved for organic materials (Tc = 18 and 33 K). Another is that C60 is an efficient sensitizer for the production of singlet oxygen ([1]O2), a highly cytotoxic form of oxygen. These results and several others warrant the int ense ongoing research on C60 and other fullerenes, notably those containing metals inside the carbon cages (endohedral metal complexes). One of our contributions to this field takes advantage of the powerful tools of organic synthesis to prepare endoh edral metal derivatives of C60. It is expected that these new compounds will have interesting and important properties, perhaps even more surprising than those of C60.
Our total synthesis scheme for endohedral complexes of C60 is based on the c onversion of compounds analogous to 1 and 2 to the fullerene framework. This unusual approach derives from mass spectroscopic studies showing that monocyclic polyacetylenes (e.g. C18, C30) convert to fullerenic oligomers (see ref. 7).
We have synthesized macrocycle 3 using a highly convergent approach. The corresponding hexabenzannelated system (C84H30), in which benzo groups replace the ethylene moieties within the enediyne units, has also been prepared. We are aiming at the preparative scale formation of fullerenes, and we are currently investigating flash vacuum pyrolysis, as well as solution phase methods (reduction, metal catalysis, etc.).
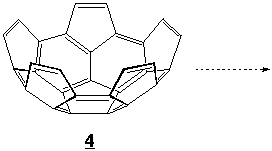
We are also working on the synthesis of several interesting bowl-shaped hydrocarbons such as 4 (C30H10). Compound 4 is exactly half of C60's framework. Linking two units of this compounds will give a clam-shell compound which should be able form transition metal complexes with structures resembling a shell enclosing a pearl. Conversions of such compounds to endohedral fullerenes will be studied by several pyrolytic and synthetic methods.
 |
 |
|---|
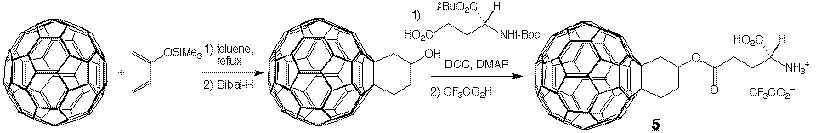
Functionalization of Fullerenes: We are actively exploring biological applications of C60 derivatives. We have recently prepared several well-characterized Diels-Alder derivatives of C60, of which some are provided with water-solubilizing functional groups (e.g. glutamate ester 4, ref. 22).
In a collaborativ
e effort with Prof. Foote in this department, we have found
that the photophysical properties of C60 are retained in these derivatives,
i.e. efficient triplet excited state formation is observed and energy transfer
to [3]O2 results in high yields of singl
et oxygen (ref. 26).
This means that such compounds are excellent potential agents for the
photodynamic therapy of cancer. In a first exciting application of these
compounds to a biological system, we have achieved the highly efficient
cleavage of DNA by
site-selective modification with the C60-linked
oligonucleotide 6 (ref. 30). Surprisingly, it is not singlet oxygen which acts
as the modifying agent but the C60 moiety reacting directly with the guanine
base, via an electron transfer mechanism.<
center>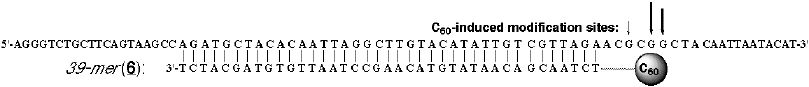
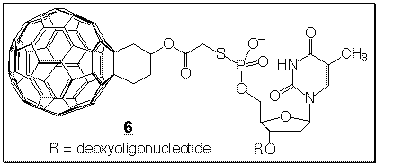
The biological chemistry of C60 has gained increasing significance in the past two years. It was found by Kenyon and Wudl th at water-soluble C60 derivatives inhibit HIV-1 protease at low micromolar concentrations. The function of this enzyme is vital to the maturation of the AIDS-inducing virus because it specifically cleaves the polyprotein precursors encoding the structural proteins and enzymes of the virus. We have started a program which uses computer assisted drug design to develop C60-based derivatives that have higher affinity and specificity in binding to HIV protease.
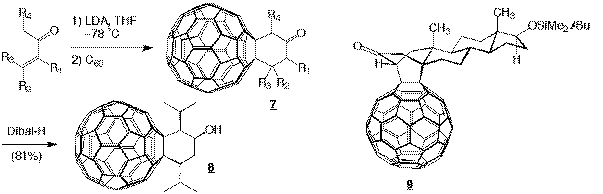
We have recently prepared the first compou nd (8) in a series of inhibitors designed to have low nanomolar binding to HIV-1 protease, a concentration which has to be reached to make any compound a viable drug. This lead was prepared utilizing the reaction of C60 with dienolates formed by LDA depr otonation of enones (ref. 27). This reaction afforded cyclohexanone-annulated C60 derivatives through a sequential bis-Michael addition mechanism. This method affords remarkably good yields of several sterically crowded monocyclic and bicyclic derivativ es of C60 (e.g. 9) and is highly stereoselective. Alcohol 8 is currently being tested on HIV-protease.
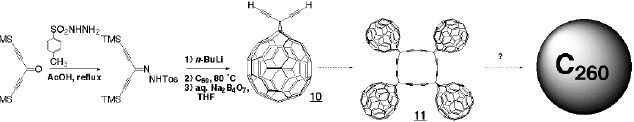
In the materials science area, we are working on the preparation of cyclic oli gomers (11) and polymeric derivatives of the diethynyl compound 10, as well as other C60 derivatives bearing conducting backbones. Because C60 readily accepts an electron to give C60[.-], it can be used as a dopant in conducting polymers. The macrocycle 11 is also envisaged to collapse to giant fullerenes, e.g. C260, which have not yet been isolated. Their properties could be intermediate between those of small fullerenes and graphite.
3.
Publications
(1) Rubin, Y.; Dick, K.; Diederich, F.; Georgiadis, T. M. "Chiral Recognition in Aqueous Solution. Search for Water-Soluble Chiral Hosts with Apolar Binding Sites", J. Org. Chem. 1986, 51, 3270-3278.
(2) Diederich, F.; Rubin, Y.; Knobler, C. B.; Whetten, R. L.; Schriver, K. E.; Houk, K. N .; Li, Y. "All-Carbon Molecules: Evidence for the Generation of Cyclo[18]carbon from a Stable Organic Precursor", Science 1989, 245, 1088-1090.
(3) Rubin, Y.; Diederich, F. "Precursors to the Cyclo[n]carbons: [4n+2]- and [4n]Annulenes w ith Unusual Stabilities", J. Am. Chem. Soc. 1989, 111, 6870-6871.
(4) Rubin, Y.; Knobler, C. B.; Diederich, F. "Precursors to the Cyclo[n]carbons: From 3,4-Dialkynyl-3-cyclobutenediones and 3,4-Dialkynyl-3-Cyclobutene-1,2-Diols to Cyclob utenodehydroannulenes and Higher Oxides of Carbon", J. Am. Chem. Soc. 1990, 112, 1607-1617.
(5) Li, Y.; Rubin, Y.; Diederich, F.; Houk, K. N. "Electronic and Structural Properties of the Cyclobutenodehydroannulenes", J. Am. Chem. Soc. 1990, 112, 1618-1623.
(6) Rubin, Y.; Knobler, C. B.; Diederich, F. "Synthesis and Crystal Structure of a Stable Hexacobalt Complex of Cyclo[18]carbon", J. Am. Chem. Soc. 1990, 112, 4966-4968.
(7) Rubin, Y., Kahr, M.; Knobler, C. B.; Diederich, F.; Wilkins, C. L. "The Higher Oxides of Carbon C8nO2n (n = 3-5): Synthesis, Characterization and X-Ray Crystal Structure; Formation of Cyclo[n]carbon Ions Cn[+](n = 18, 24) and Cn[-](n = 18, 24, 30), and Higher Car bon Ions Including C60[+] in Laser Desorption Fourier Transform Mass Spectrometric Experiments", J. Am. Chem. Soc. 1991, 113, 495-500.
(8) Ajie, H.; Alvarez, M. M.; Anz, S. J.; Beck, R. D.; Diederich, F.; Fostiropoulos, K.; Huffman, D. R. ; Kratschmer, W.; Rubin, Y.; Schriver, K. E.; Sensharma, D.; Whetten, R. L. "Characterization of the Soluble All-Carbon Molecules C60 and C70", J. Phys. Chem. 1990, 94, 8630-8633.
(9) Arbogast, J. W.; Darmanyan, A. P.; Foote, C. S.; Rubin , Y.; Diederich, F.; Alvarez, M. M.; Anz, S. J.; Whetten, R. L. "Photophysical Properties of C60", J. Phys. Chem. 1991, 95, 11-12.
(10) Allemand, P.-M.; Koch, A.; Wudl, F.; Rubin, Y.; Diederich, F.; Alvarez, M. M.; Anz, S. J.; Whetten, R. L. "C60 and C70 Have the Same Redox Behavior", J. Am. Chem. Soc. 1991, 113, 1050-1051.
(11) Allemand, P.-M.; Srdanov, G.; Koch, A.; Khemani, K.; Wudl, F.; Rubin, Y.; Diederich, F.; Alvarez, M. M.; Anz, S. J.; Whetten, R. L. "The Unusual Electron Spin Resonance of Fullerene C60[*-]", J. Am. Chem. Soc. 1991, 113, 2780-2781.
(12) Diederich, F.; Ettl, R.; Rubin, Y.; Whetten, R. L.; Beck, R. D.; Alvarez, M. M.; Anz, S. J.; Sensharma, D.; Wudl, F.; Khemani, K. C.; Koch, A. "Th e Higher Fullerenes: Isolation and Characterization of C76, C84, C90, C94, and C70O, an Oxide of D5h-C70", Science 1991, 252, 548-551.
(13) Snyder, E. J.; Anderson, M. S.; Tong, W. M.; Williams, R. S.; Anz, S. J.; Alvarez, M. M.; Rubin, Y.; Diederich, F.; Whetten, R. L. "Atomic Force Microscope Studies of Fullerene Films: Highly Stable C60 fcc (311) Free Surfaces", Science 1991, 253, 171-173.
(14) Tong, W. M.; Ohlberg, D. A. A.; You, H. K.; Williams, R. S.; Anz, S. J.; Alvarez, M. M.; Whetten, R. L.; Rubin, Y.; Diederich, F. "X-Ray Diffraction and Electron Spectroscopy of Epitaxial Molecular C60 Films", J. Phys. Chem. 1991, 95, 4709-4712.
(15) Rubin, Y.; Knobler, C. B.; Diederich, F. "Tetraethynylethen e", Angew. Chem. 1991, 103, 708-710; Angew. Chem. Int. Ed. Engl. 1991, 30, 698-700.
(16) Rubin, Y.; Lin, S. S.; Knobler, C. B.; Anthony, J.; Boldi, A. M.; Diederich, F. "Solution-Spray Flash Vacuum Pyrolysis: A New Method f or the Synthesis of Linear Poliynes with Odd Numbers of C[[equivalence]]C Bonds from Substituted 3,4-Dialkynyl-3-cyclobutene-1,2-diones", J. Am. Chem. Soc. 1991, 113, 6943-6949.
(17) Whetten, R. L.; Alvarez, M. M.; Anz, S. J.; Schriver, K . E.; Beck, R. D.; Diederich, F. N.; Rubin, Y.; Ettl, R.; Foote, C. S.; Darmanyan, A. P.; Arbogast, J. W. "Spectroscopic and Photophysical Properties of the Soluble Cn Molecules, n = 60, 70, 76/78, 84", Mat. Res. Soc. Symp. Proc. 1991, 206, 639-650.
(18) Diederich, F.; Rubin, Y. "Synthetic Approaches toward Molecular and Polymeric Carbon Allotropes", Angew. Chem. 1992, 104, 1123-1146; Angew. Chem. Int. Ed. Engl. 1992, 31, 1101-1123 (Review).
(19) Rubin, Y.; Khan, S.; Freedberg, D. I.; Yeretzian, C. "Synthesis and X-Ray Structure of a Diels-Alder Adduct of C60", J. Am. Chem. Soc. 1993, 115, 344-345.
(20) Khan, S. I.; Oliver, A. M.; Paddon-Row, M. N.; Rubin, Y. "Synthesis of a Rigid "Ball-and- Chain" Donor-Acceptor System through Diels-Alder Functionalization of Buckminsterfullerene (C60)", J. Am. Chem. Soc. 1993, 115, 4919-4920.
(21) Baak, M.; Rubin, Y.; Franz, A.; Stoeckli-Evans, H.; Bigler, L.; Nachbauer, J.; Neier, R. "An U nexpected Tandem Reaction between (N-Butadienyl-N-alkylketene)-N,O-Trimethylsilylacetas of Propionamide and Activated Dienophiles like N-Phenylmaleimide or Acryloyl Chloride" Chimia 1993, 47, 233-240.
(22) An, Y.-Z.; Anderson, J. L.; Rubin, Y. "Synthesis of [[alpha]]-Amino Acid Derivatives of C60 from 1,9-(4-Hydroxycyclohexano)buckminsterfullerene" J. Org. Chem. 1993, 58, 4799-4801.
(23) Rubin, Y.; An, Y.-Z.; Schaller, C.; McElvany, S. W. "Syn thesis and Characterization of Diethynylmethanobuckminsterfullerene (C65H2), a Building Block for Macrocyclic and Polymeric Carbon Allotropes" J. Org. Chem. 1994, 59, 2927-2929.
(24) Anderson, H. L.; Faust, R.; Rubin, Y.; Diederich, F. "F ullerene-Acetylene Hybrids: On the Way to Synthetic Molecular Carbon Allotropes", Angew. Chem. Int. Ed. Engl. 1994, 33, 1366-1368.
(25) Diederich F; Rubin Y; Chapman, O. L.; Goroff N. S. "Synthetic Routes to the Cyclo[n]carbons", Helv. Chim. Acta 1994, 77, 1441-1457.
(26) Anderson, J. L.; An, Y.-Z.; Rubin, Y.; Foote, C. S. "Photophysical Characterization and Singlet Oxygen Yield of a Dihydrofullerene", J. Am. Chem. Soc. 1994, 116, 9763-9764.
(27) Ganapathi, P. S.; Friedman, S. H.; Kenyon, G. L., Rubin, Y. "Sequential "bis-Michael" Additions of Dienolates with C60: Rapid Access to Sterically Congested Buckminsterfullerene Derivatives with Defined Stereochemistry", J. Org. Chem. 1995, 60, 2954-2955.
(28) An, Y.-Z.; Ellis, G. A.; Viado, A. L.; Rubin, Y. "A Methodology for the Reversible Solubilization of Fullerenes", J. Org. Chem. 1995, in press.
(29) Rubin, Y. "Recent Aspects of the Functionalization Chemistry of Bu ckminst erfullerene (C60): Preparation of New Materials and Compounds of Biological Interest", NATO ASI Series 1995, in press
(30) Chen, C.-h. B.; An, Y.-Z.; Sigman, D. S.; Foote, C. S.; Rubin, Y. "Sequence-Specific Modification of Guanosine in DNA by a Dihydrofullerene-linked Deoxyoligonucleotide: Evidence for a non-Singlet Oxygen Mechanism", Tetrahedron 1995, in press.
Department * Department Faculty* Current Organic Research* Rubin Biography* Organic Research Interests ]
Last Revision: 08/23/95 // burns@chem.ucla.edu