|
|
Email: mfh@chem.ucla.edu |
Condensed version of the 79th Faculty Research Lecture Presented by Professor M. Frederick Hawthorne The title of my lecture, "From Mummies to Rockets and on to Cancer Therapy," was created on a train somewhere near Heidelberg, Germany, about six weeks ago. I needed a title, and that just came to mind and stuck. It really is an excuse to talk about boron chemistry, something that has been an integral part of my life since about 1956.The Periodic Table, the chart illustrating the organization of the various elements, shows boron as the fifth element. Boron is the nearest neighbor to carbon on the right, aluminum below it and beryllium on the left. Beryllium's a metal; carbon's a nonmetal. Carbon plays a tremendous role in biology and, in fact, all aspects of our life. And boron, right next to it, would be expected to have a similarly important role to play. Today it's my job to try to convince you that perhaps this is true. The chemistry of boron is dictated by its position as the fifth element; as such, it has a nucleus which has a plus-five charge. It has five protons in the nucleus. It has five electrons around the nucleus. This family of electrons determines the chemical reactivity of the atom. 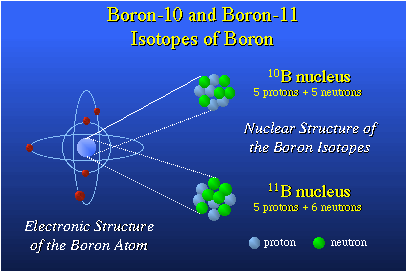
There are two kinds of boron nuclei. These are the B-10 nucleus composed of five protons and five neutrons and the B-11 nucleus which has five protons and six neutrons. So these differ by simply the presence or absence of a neutron. A neutron has no charge; consequently, both of these are plus-five nuclei and they have five electrons around them and they are both isotopes of the element boron. The world has quite a lot of boron-10 and boron-11.Boron especially likes to combine with oxygen. Boron occurs in nature in the form of oxides, usually borates, which are of volcanic origin. The boron oxide salts were washed down into salt flats now rich in borax.One of the cottage industries in early Egypt was running funeral homes, doing people up nicely with linen. The mummification process depended upon a salt known as natron that came from the Natron Valley in Egypt. Natron contained borates as well as other common salts such as sodium bicarbonate, sodium chloride and the like. Natron was a highly prized substance and it was rather costly for the people to purchase, so a really good mummy job had lots of natron spent on it and one that wasn't quite so ritzy had less natron. (The difference showed years later.) Natron was used to desiccate the deceased and it also was a bacteriostat and a fungistat. Later on, boron was introduced into Roman glass and also used as a welding flux in China. Marco Polo brought it to Italy, and it was used by artisans in Italy at the time. 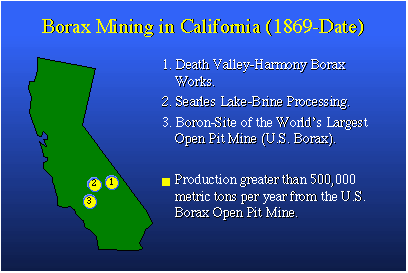
Boron minerals were discovered in what we now know as Southern California about 1869, and there are many, many localities in the state where boron is available, but three are worth mentioning. The Harmony Borax Works in Death Valley was one of the original sources of borate. Searles Lake is a dry lake that hasn't quite dried yet. It has brine rich in boron that's processed for boric acid. And there is the world's largest open pit mine, owned by U.S. Borax. About half of the mined borax used each year in the world comes from this site. That's a lot of boron: about 500,000 metric tons per year.Perhaps you're familiar with 20 Mule Team Borax, the trademark of U.S. Borax. The 20-mule teams at Harmony Borate Works in Death Valley would take a wagon of borate ore 165 miles to Mojave, Calif., to the railhead, at which point it was transferred to rail cars and shipped out for industrial purposes. The story is that after many, many years of operation, not a single mule was lost. It was all very humane.In the 1950s U.S. Borax, by way of advertising, produced a TV series called "Death Valley Days." It was a Western-type drama in which the good guy always won. The good guy was Ronald Reagan. One could say the program's popularity contributed somewhat to his popularity as a human being and as an actor, and certainly helped him in his political career. Now, let's talk a little bit about boron itself. We know boron likes to combine with oxygen and form borates such as borax, boric acid and boric anhydride. We know carbon, next to boron on the Periodic Table, combines readily with oxygen to form carbon monoxide and carbon dioxide. We also know carbon combines with hydrogen to form hydrocarbons, such as the gasoline that goes into our gasoline tanks. 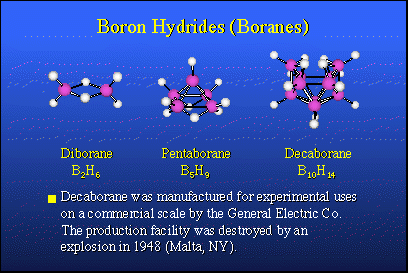
In nature, there are no equivalent boron-hydrogen compounds and there is no petroleum analog for boron. Therefore, about 1910, Alfred Stock in Karlsruhe, Germany, took it upon himself to explore the possibility of preparing boron hydrogen compounds, so-called boron hydrides, or boranes, which would be analogous to hydrocarbons. He succeeded in making small quantities of very expensive, very rare compounds. Their chemistry was not well understood until about 1950. The nice thing about these materials is that when they burn, they release large quantities of heat and they would make excellent fuels if one had sufficient quantities of them.In 1946, following the conclusion of World War II, Project Hermes was organized by the U.S. Army. The purpose was to synthesize boron hydrides in commercial quantities and perhaps use them as fuels in rockets. The heat of combustion of boron hydrides is typically about 30,000 BTUs per pound, while a hydrocarbon fuel, such as JP4 or kerosene, was about 18,000 BTUs. This was a tremendous step up in potential energy content, and it was attractive to people designing rocket engines and weapon systems.Accordingly, General Electric Company undertook a research and development program in Malta, N.Y., which actually made commercial quantities, several hundred pounds of diborane, pentaborane and decaborane. These more stable boron hydrides are the contributions of Alfred Stock. All went well until about 1948, when the plant was cleaned and washed out with carbon tetrachloride, not realizing that carbon tetrachloride and decaborane form an explosive mixture equivalent to nitroglycerin. The plant was blown away. So, live and learn. Anton Burg at USC had warned them that this might happen. They did it nonetheless.That didn't change things and the rocket program went ahead. In the meantime, a new bomber was designed, the B-70 Valkyrie, an intercontinental bomber capable of reaching the Soviet Union and returning to the United States without midair refueling. To do this, it required boranes as fuels to operate the turbojet engines. Millions and millions of dollars were put into a fuel program as well as the bomber itself.After about 10 years of research, it was found that you just could not make a turbine engine strong enough to stand up to the boron oxide and boron carbide products of combustion formed from the fuels, so this project was dropped and the B-70 reverted to hydrocarbon fuels. The rocket fuel program floundered. The problem was that the solid boron oxides present in the combustion products of the rocket motor, interfered with the expected thermodynamics. So boranes were not super fuels for rockets and that program was dropped. An unexpected finding, however, was that solid propellants based upon boron hydrides have a very high burning rate and this makes them attractive for anti-ballistic missile defense rockets and other things that require rapid response. That application still exists today, although nothing is really being done about it. 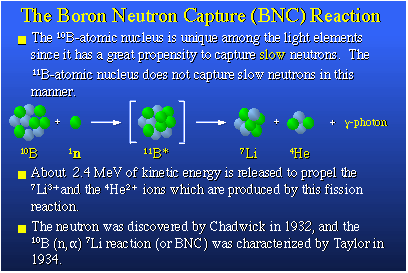
I started by telling you there were two isotopes of boron, boron-10 and boron-11. Boron-10 is the only light element with an extremely high propensity to bind with a slow neutron that might present itself to the boron nucleus. That reaction is shown here. The boron-10 nucleus really likes neutrons that are moving slowly, so-called thermal neutrons. The boron-10 will pick up this neutron and form an excited boron-11 nucleus. This nucleus is unstable and it fissions, it actually blows up producing two heavy particles, a lithium-7 nucleus and a helium-4 nucleus, plus a gamma photon. About 2.4 million electron volts of kinetic energy are released in this process and that appears principally as translational energy in the two heavy particles, the lithium and the helium nuclei. They are heavy and they're moving rapidly. That makes them deadly to cells if they are created in tissue. I should give credit to the neutron discoverer, Chadwick, in England in 1932. The boron-10 neutron capture reaction was characterized first by Taylor and Fermi and others about 1934, and its application to medicine, which I will tell you about, was proposed in 1936. 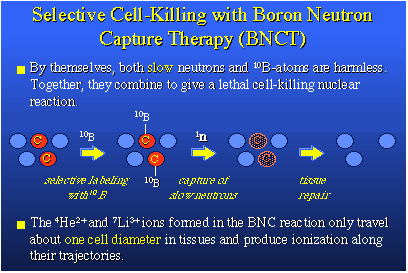
How would you apply this to medicine? The answer was produced by Locher, a physicist. The idea is if you have a tumor containing both normal cells, in blue, and cancer cells, in red and yellow, and the chemist by some hook or crook could selectively attach boron-10 to only the cancer cells, then you have boron-10 localized in the cells you wish to destroy. You then irradiate this whole region with slow neutrons, which are easily captured by boron-10, and then the boron neutron capture reaction, or BNC, should occur. The BNC will kill the cells to which the boron-10 target species was attached. Then the body repairs itself and these dead cells disappear. The nice thing about this process is that the ionization tracks of the helium and lithium ions produced from the fission reaction are only about one cell diameter in length and quite localized. The processes which lead to actual cellular death are the result of ionization tracking. The ions produced from the fission reaction are energetic and heavy on an atomic scale and they plow through the cell and virtually tear it to pieces. 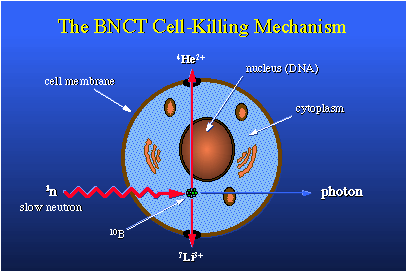
Of course, this is what we want to happen. This is an idealized case in which we have a typical cell. Here's the nucleus with DNA, and we'd really like to damage that DNA by ionization tracking. To do so would kill the cell outright. Let's just place a boron-10 nucleus at this point. A slow neutron diffuses in, becomes captured, and the BNC reaction occurs. This nucleus fissions into a helium ion, which is an alpha particle, and into a lithium-7, and these ions go opposite directions. The photon is released. But in going through the cell you see that the helium ion passes through the nucleus, which should be quite destructive to DNA inside that nucleus. Other cellular components could be damaged as well. For therapy we want to put about 109 boron-10 atoms in each and every cell we wish to kill. This amounts to about 30 micrograms of boron-10 per gram of tumor.To make all this work, you need about 1012 neutrons per square centimeter, and this means a person undergoing therapy using this method would require something like 20 to 30 minutes' exposure to a neutron source which delivers about 109 thermal neutrons per square centimeter per second. That's an easily obtainable value.BNCT is a two-component, binary, radiation therapy, which is unique. There's the neutron, which can be turned on and off, and the boron, which if employed correctly, is perfectly innocuous. The therapy is under development for treatment of glioblastoma multiform, a very deadly brain cancer. In the United States there are probably 7,000 new candidates for this therapy each year. BNC therapy also is being considered for use in lung and prostate cancers, of which there are many, many more new cases each year. The neutron of choice for the brain cancer work is an epithermal neutron. It has kinetic energy somewhat less than one kilo-electron volt, one keV. Epithermal neutron beams can come from nuclear reactors or charged particle accelerators. These particle accelerators could be set up in clinics located in urban surroundings and require the patient to venture into the countryside to utilize a nuclear reactor. 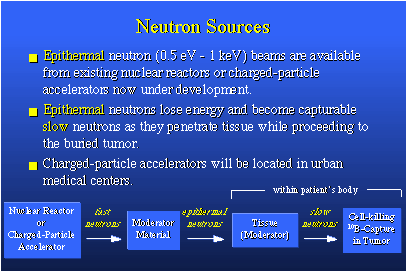
When they emerge from their source, epithermal neutrons easily penetrate tissue and in so doing slow down and become thermal neutrons, and capturable. Here's a nuclear reactor, or charged particle accelerator, putting out fast neutrons. Fast neutrons go through a material, called a moderator, which is rich in hydrogen. It could be a tank of water. This slows down the fast neutrons to the epithermal range. If you're after a deeply buried tumor, let's say six, eight or 10 centimeters inside the skull, the tissue between the outside of the skull and the tumor itself serves as a moderating material and slows the epithermals to the slow speed required for capture. At the tumor, capture occurs and everything works. 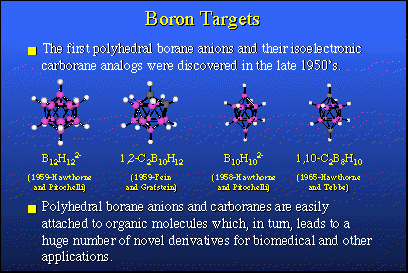
The neutrons required for therapy are not too difficult to obtain. What is a bit difficult to get your hands upon are the proper chemicals, proper boron-10 containing agents which serve as neutron target species. Fortunately, in the past we did have a tremendous effort in the borane fuels, with rockets and airplanes, and from that research large families of new borane derivatives were obtained and all sorts of new discoveries were made. These are some of the compounds that were discovered in the late '50s. If you note, these species resemble bird-cages. They're all closed up. In fact, they are regular polyhedra, many of them. They have spatial properties and they are particularly stable chemically. They do not oxidize in air and they do not hydrolyze readily in water. They are quite nice to work with and they're virtually nontoxic.So, we can hook these boron compounds up with organic molecules which leads to huge numbers of derivatives some of which could have very interesting biomedical applications. That kind of work has been going on at UCLA during the last 30 years. In about 1965 we expanded this area quite a bit more by finding that one could introduce metals from other parts of the Periodic Table into polyhedral borane structures, and so we and others have visited most of the Periodic Table and introduced atoms that were not carbon atoms, nor were they boron atoms, but they were metals like iron and cobalt, nickel, silicon, aluminum and many others. 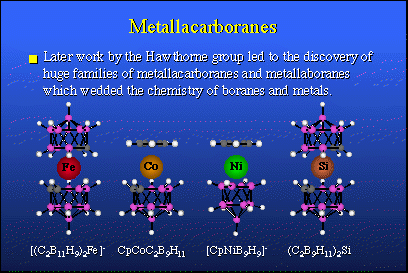
The challenge that BNC therapy brings to the chemist is the requirement to do two things at once. You have to develop polyhedral borane chemistry, find new reactions and new ways to do things, and you must apply these concepts to the design and synthesis of new materials. We need selectivity in the uptake of boron compounds by cancer cells, rather than by normal cells. This is not an easy thing to predict and obtain. Also, you must accumulate at least 20 micrograms of boron-10 per gram of tumor in the cancer cells. This would be a useful concentration. It must go into the cancer cell and be retained there sufficiently long for you to apply neutron radiation to that area in order to accomplish therapy.How do we proceed in our laboratory? Well, first one conceives an idea for a new type of molecule. We then design what it should look like and how to make it. Then we prepare it in a synthesis laboratory and then it's characterized chemically and physically.At this point the chemist's job at UCLA is finished. The compound is now transported to the Washington State University School of Veterinary Medicine and our colleague there, Dr. Patrick Gavin, who's in charge of our evaluation program, takes over. The compound is administered to mice bearing tumors. The more effective compounds are then synthesized on a larger scale and injected into large animals with natural tumors.These animals then are treated with the epithermal beam at Washington State University which maintains such a neutron source for this purpose. This procedure completes animal work. From here the results would go to the FDA and enter clinical evaluation in a normal sense. There are two areas of particular interest to us at UCLA that have withstood the test at WSU. The first of these is the tumor cell-selective unilaminar liposomes which contain a lot of boron. The nice thing is that once injected in an animal with a tumor liposomes will find their way to the tumor, enter the tumor cells and thereby give you a pipeline from the chemist's laboratory bench right into the tumor cell of the patient. This is something we've all wanted. 
Item two is DNA-binding phosphate diesters based upon carborane chemistry. These are DNA binding because we fix them up with chemical hooks, so to speak, which selectively seek out and bind the tumor cell's DNA. The liposome is a tiny vesicle, a spherical shell composed of a phospholipid bilayer, derived from a molecule which has a water-soluble end and two greasy, water-insoluble tails that tend to associate with each other, leaving the polar ends to associate with water. The unilamellar liposome has water on the inside, water on the outside, and it swims in water. It is quite small, less than a hundred nanometers in diameter, or about one one-thousandth the diameter of a red blood cell. Yet it has this beautifully defined structure. 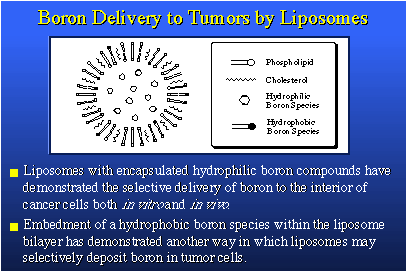
The compounds we wish to deliver to cancer cells can be placed inside the liposome in the his aqueous center. These would be water-soluble compounds that are hydrophilic. Compounds that are hydrophobic or lipophilic can be placed in the bilayer. They can actually be dissolved in the bilayer lipid material and carried in that fashion. We therefore have two compartments in the normal unilamellar liposome for the transport of boron compounds. This is a slice of a liposome, showing you the bilayer with the aqueous center and water-soluble borane species dissolved in water on the inside. In the bilayer, the dark spots represent boron species containing a hydrophobic tail, which allows them to be incorporated in the bilayer. Liposomes having materials on the inside have been shown to deliver those materials to the interior of cancer cells in both in vitro and in vivo experiments. 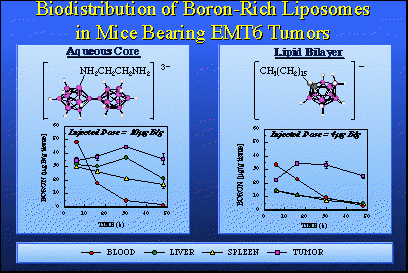
The species on the left has 20 boron atoms in it. Those are the mauve spheres. It's a three-minus ion. It forms a very soluble sodium salt. This salt, when dissolved in water, can be placed on the inside of the liposome and then administered to animals, in this case mice bearing EMT-6 adenocarcinoma tumors. You will note the tumor is a mauve square and the tumor line shows the build-up of boron is really quite good. We need something like 20 to 30 parts per million to have a therapeutic dose and here we are peaking out after about 30 hours at 40 parts per million and we're only injecting 10 micrograms of boron per gram of animal. We are actually amplifying the average concentration injected by a factor of four. The nice thing about this is that the blood clears quite readily. You see that the wash-out of blood, liver and spleen are about as you'd expect. This is a good boron agent. We're very excited by this, and it's going into larger animals very shortly.On the right we see a compound that is accommodated in the bilayer. This is an anion, actually a negatively charged group attached to a greasy hydrocarbon tail. It fits nicely in a bilayer membrane. The injected dose is four micrograms of boron per gram of mouse. It's not very much. Now we're again getting above 30 parts per million in tumor even though we're putting in very low injected doses. We observe very good clearance of boron from tissues other than tumor. We're very excited by both of these sets of data. The other thing we're quite excited about is some work that's been pushed by Dr. Bob Kane, in our laboratory, involving what we simply call "trailers" because the proper name is quite cumbersome; oligomeric phosphate diesters. They are actually short chain polymers and they are phosphate esters derived from a phosphate ester linkage and a diol. We synthesize the phosphate diester of a diol and this gives us an oligomer in which we have several of these groups held one to the other n times. We can make these oligomers any size we want by selecting the value of n. 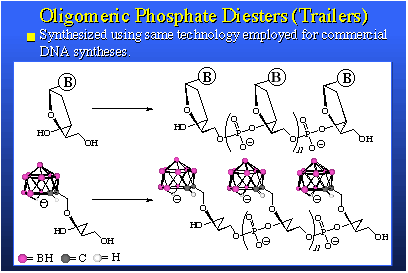
Above here is a stylized view of a DNA molecule. Machines and technology exist to synthesize strings of DNA. Because our starting materials look and behave like DNA, we can use this same technology to make oligomers; trailers. We can put strings of actual DNA onto our oligomers, and vice versa. This is very simple and the versatility that one obtains in the structure of the products is immense. The trailers we synthesize are boron rich, water soluble, bio-compatible, nontoxic, and they present tremendous diversity of structure and secondary chemical function. They can be used in several ways. We can use them as free agents, that is, simply inject them in the animal and let them circulate by themselves, or they can be incorporated within a liposome. Or they can be attached to biomolecules that are themselves targeted for receptor sites in the tumor cell and its DNA. This would involve biomolecule carriers such as hormone surrogates, DNA intercalators, peptides or DNA segments themselves. So, this boron-delivery method has a tremendous scope, both in chemical structure and in use. We're quite excited by our preliminary results. The performance is, so far, astounding. 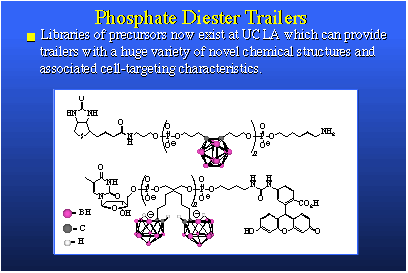
Heretofore, today, we've talked about boron neutron capture therapy using epithermal neutrons. Not too many years ago, fast neutrons were explored as therapeutic radiation and they are still used. They're now used clinically at the University of Washington Medical Center. Fast neutrons having energies, say 25 to 50 million electron volts, are so energetic that they behave like photon beams, which can be shaped. They can be precisely aimed at a target -- a deeply buried tumor in a patient -- and hit that target with great accuracy. Such neutron beams are used therapeutically for certain types of cancer. Prostate cancer is one type. Lung cancer is another. 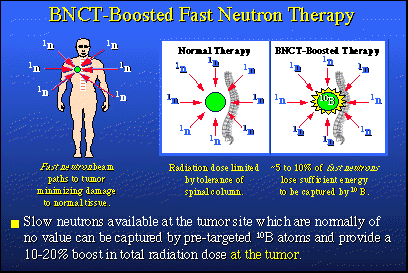
So let's say this human being has a lung tumor, which is the green spot in the illustration. The normal treatment would be to go after this tumor with fast neutrons down a variety of channels. On a specific day, you might come in from one direction and hit the tumor. A few days later, you might come in another way and hit the same tumor using another pathway.Now, fast neutrons are quite deadly to tissue. But in each of these neutron irradiation sessions, you're attacking the tumor and you're killing it. You are controlling it. However, you're also doing some damage to normal tissue as neutron irradiation proceeds, but this collateral damage is being spread around so it's not going to be irreversible in any sense. Nonetheless, there's a limit to how many times you can apply fast neutrons. That limit, in the case of a lung cancer, is often determined by the proximity of the tumor to the spinal column and the unavoidable collateral damage that could be done to the spinal column by these fast neutron beams when they are applied. Professor George Laramore at the University of Washington Cancer Center thought a lot about this fast neutron radiation process and also coupled it with the boron-10 neutron capture idea. Because he is a Ph.D. physicist as well as a physician, he modeled fast neutron therapy and found that during this process about 5 to 10 percent of the fast neutrons employed have been slowed down sufficiently by the time they reach the tumor to be captured by a boron-10 nucleus. In the new procedure, before we begin the normal fast neutron treatment, we load the tumor with boron-10. We go ahead and give normal fast neutron treatments, but the slow neutrons that are produced at the tumor and normally thrown away can be captured by the waiting boron-10. That capture process gives us a 10 to 20 percent boost in total radiation dose at the tumor. 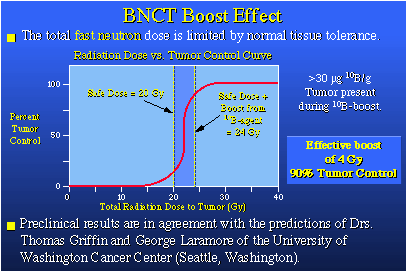
The enhanced radiation dose which arises from the BNC boost should markedly improve tumor control due to the shape of the tumor control versus radiation dose curve. This is an exciting prospect. View All |