|
|
Email: mfh@chem.ucla.edu |
Condensed version of the 79th Faculty Research Lecture Presented by Professor M. Frederick Hawthorne The title of my lecture, "From Mummies to Rockets and on to Cancer Therapy," was created on a train somewhere near Heidelberg, Germany, about six weeks ago. I needed a title, and that just came to mind and stuck. It really is an excuse to talk about boron chemistry, something that has been an integral part of my life since about 1956.The Periodic Table, the chart illustrating the organization of the various elements, shows boron as the fifth element. Boron is the nearest neighbor to carbon on the right, aluminum below it and beryllium on the left. Beryllium's a metal; carbon's a nonmetal. Carbon plays a tremendous role in biology and, in fact, all aspects of our life. And boron, right next to it, would be expected to have a similarly important role to play. Today it's my job to try to convince you that perhaps this is true. The chemistry of boron is dictated by its position as the fifth element; as such, it has a nucleus which has a plus-five charge. It has five protons in the nucleus. It has five electrons around the nucleus. This family of electrons determines the chemical reactivity of the atom. 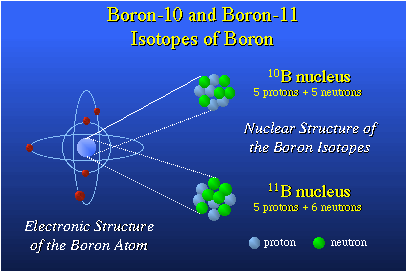
There are two kinds of boron nuclei. These are the B-10 nucleus composed of five protons and five neutrons and the B-11 nucleus which has five protons and six neutrons. So these differ by simply the presence or absence of a neutron. A neutron has no charge; consequently, both of these are plus-five nuclei and they have five electrons around them and they are both isotopes of the element boron. The world has quite a lot of boron-10 and boron-11.Boron especially likes to combine with oxygen. Boron occurs in nature in the form of oxides, usually borates, which are of volcanic origin. The boron oxide salts were washed down into salt flats now rich in borax.One of the cottage industries in early Egypt was running funeral homes, doing people up nicely with linen. The mummification process depended upon a salt known as natron that came from the Natron Valley in Egypt. Natron contained borates as well as other common salts such as sodium bicarbonate, sodium chloride and the like. Natron was a highly prized substance and it was rather costly for the people to purchase, so a really good mummy job had lots of natron spent on it and one that wasn't quite so ritzy had less natron. (The difference showed years later.) Natron was used to desiccate the deceased and it also was a bacteriostat and a fungistat. Later on, boron was introduced into Roman glass and also used as a welding flux in China. Marco Polo brought it to Italy, and it was used by artisans in Italy at the time. 
|