|
|
Email: mfh@chem.ucla.edu |
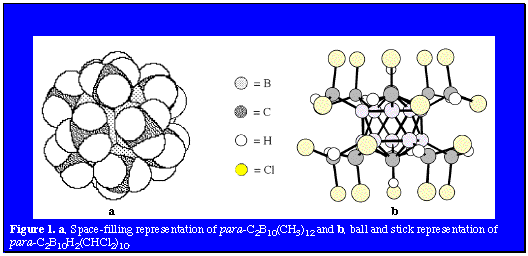
Camouflaged Polyhedral Species [pdf] The three isomers of the icosahedral C2B10H12 (1,2- or ortho-; 1,7- or meta-; and 1,12- or para-) carboranes and the isoelectronic polyhedral borane dianion B12H122- are uniquely suited to play the role of building-blocks in the construction of molecular scaffolding and stereochemically rigid platforms. These versatile components often provide sites for further functionalization of the macrostructure or to add other interesting chemical properties. This versatility has lead to the formation of several different types of structural motifs, including carborods, mercuracarborands, carboracycles, and camouflaged carboranes and polyhedral borane anions. This section of the web site will focus on the latter of these structural types, the camouflaged carboranes and polyhedral borane anions. The term camouflaged carborane or polyhedral borane describes a molecule in which all, or very nearly all, the B-H vertices have been substituted by a typical organic substituent. The resulting polyhedral borane derivative, which has a borane core stabilized by multicenter electron delocalization, is shrouded by a periphery of organic substituents. Hence, camouflaged carboranes demonstrate chemical properties indicative of their hybrid nature between that of the thermally and chemically stable carboranes and boranes and the substitutionally versatile aliphatic hydrocarbons.
Another application which is of particular interest is the possible adoption of these camouflaged icosahedral carboranes as the bases of an inventory of large, hydrophobic and biodegradation-resistant pharmacophore groups and their potential functionalization for use in drug discovery. A comparison of the calculated van der Waals diameters of dodecamethyl-para-carborane (990 pm), decakis(dichloromethyl)-para-carborane (1440 pm) and a well-known hydrophobe, C60 (1070 pm) shows the similar volumes of these three molecules. In order to permit either of these camouflaged carboranes to be used as pharmacophore groups, reactions which provide further functionalization of the carboranes needed to be developed. The photochlorination results demonstrate that reactions characteristic of aliphatic hydrocarbons can be utilized to functionalize the organic shroud surrounding the delocalized polyhedral cage bonding of the carborane scaffolding. This hydrocarbon-like reactivity was further exploited through adaptation of the Barton photochemical oximation reaction. |