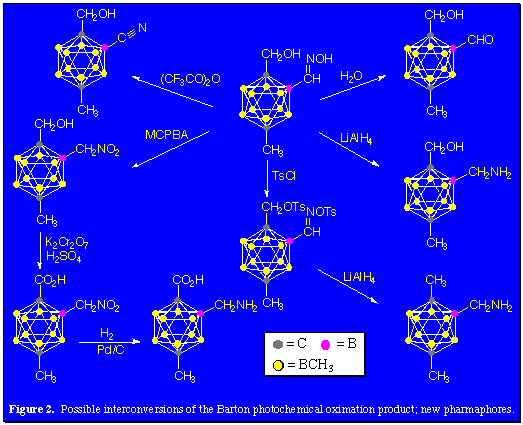
By focusing on functionalization at one of the ten available methyl groups of per-B-methyl-para-carborane, isomerically pure monofunctional products can be prepared. The Barton reaction is an example of an intramolecular regiospecific radical reaction capable of activating methyl C-H bonds. The rigid geometry of an appropriately carbon-substituted deca-methyl-paracarborane is ideally suited for the Barton reaction which through the photochemical decomposition of a nitrite ester function produces NO and an alkoxy radical in close proximity to a C-H bond. The nitrite-substituted carborane was prepared from the reaction of deca-B-methyl-1-hydroxymethyl-1,12-dicarba-closo-dodecaborane(12) and nitrosyl chloride in pyridine in excellent yield, 95%. The ensuing photolysis of this product in benzene produces the boron-substituted hydroximino alcohol in 42% to 25% yields. Figure 2 shows a few of the organic conversions which are feasible using this versatile hydroxyimino alcohol-substituted camouflaged carborane.
Having successfully developed a synthetic approach to decamethyl- and dodecamethyl-para-carboranes, it became feasible to attempt the similar methylation of 1,2- and 1,7-C2B10H12 and at the same time perhaps increase the yields of decamethyl- and dodecamethyl-para-carborane. A new procedure employing AlCl3 in neat CH3I solvent easily methylated para-carborane in high yield by substitution of all available B-H vertices. This procedure allowed for the preparation of 4,5,7,8,9,10,11,12-octamethyl-1,2-dicarba-closo-dodecaborane(12) and 4,5,6,8,9,10,11,12-octamethyl-1,7-dicarba-closo- dodecaborane(12) derivatives of 1,2- and 1,7-C2B10H12 after 50 h using either 1 or 10 equivalents of AlCl3. In both cases the two equivalent B-H vertices nearest the 1,2- or 1,7- pairs of C-H vertices escaped reaction.
The class of camouflaged carboranes was further extended to include a nido-carborane anion with the preparation of 1,2,4,5,6,9,10,11-octamethyl-7,8-dicarba-undecaborate(-1) through the base-mediated deboration of closo-1,2-C2B10Me8H4. Not surprisingly, the octa-B-methylated, closo-carborane was quite resistant to degradation. However, complete conversion to the corresponding nido-carborane anion was accomplished by treatment of the closo-carborane with an excess of potassium ethoxide in dimethoxyethane solution in a high-pressure vessel at 200°C for 14 hours.
When prontonated, this camouflaged nido-carborane demonstrates enhanced stability over that of the similar, non-camouflaged, nido-7,8-C2B9H13. While simple heating of nido-7,8-C2B9H13 results in dehydrogenation to form closo-1,8-C2B9H11, the camouflaged nido-carborane must be passed over a bed of silica in order to induce dehydrogenation. Interestingly, nido-7,8-C2B9H13 is inert in the presence of silica. The resulting crystalline product is a mixture of two isomers of closo-1,8-C2B9Me8H3, resulting from the differing positions of the unique B-H vertex. From this isomeric mixture, a single pure compound was prepared by methylation of the single B-H vertex present in each isomer to produce 2,3,4,5,6,7,9,10,11-nonamethyl-1,8-dicarbaundecaborane(11), closo-1,8-C2B9Me9H2, by reaction with MeI/AlCl3 at the reflux temperature for 8 hours, in 95% yield.
Page 1 2 3 4  |
